Gufic Biosciences Walk-In – Production & QA Roles


Gufic Biosciences Limited
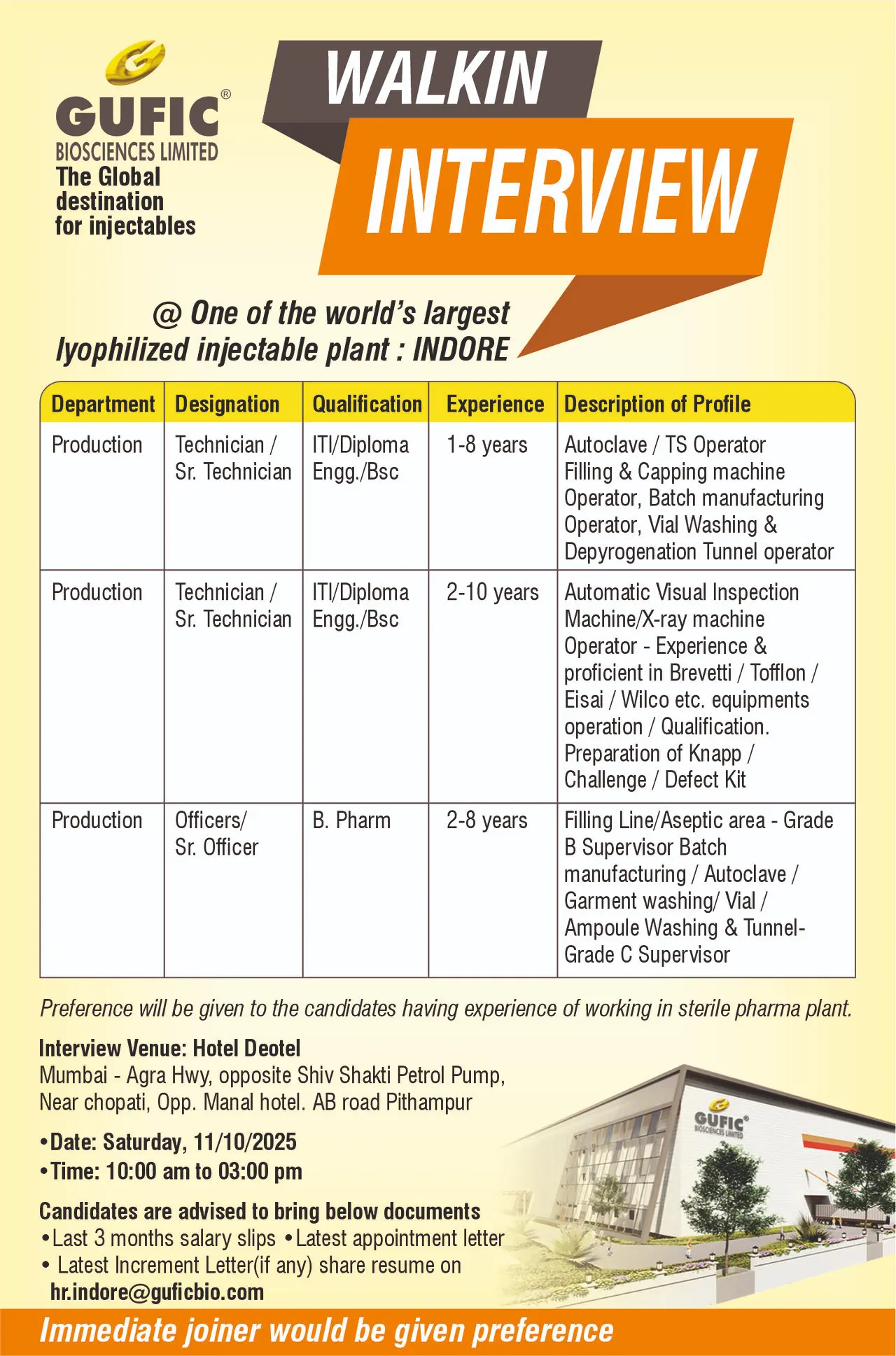
Gufic Biosciences Walk-In – Production & QA Roles, Indore
Gufic Biosciences hiring Production & QA Officers, Technicians. Walk-In Drive on 11th Oct 2025 in Indore. Apply with documents. sydney sweeney tits Gufic Biosciences Ltd, one of the world’s largest lyophilized injectable plants, is conducting a Walk-In Recruitment Drive for talented professionals passionate about pharmaceutical manufacturing and quality assurance. This is a unique opportunity to join a globally recognized organization that emphasizes innovation, operational excellence, and regulatory compliance.
Company Overview
Gufic Biosciences Limited is a global leader in injectable pharmaceuticals with a state-of-the-art lyophilized manufacturing facility in Indore. Renowned for delivering high-quality sterile products, Gufic prioritizes patient safety, regulatory compliance, and continuous innovation. Employees benefit from a structured work environment, professional development opportunities, and exposure to cutting-edge pharmaceutical technologies.
Job Roles & Responsibilities
Production Department
Technician / Senior Technician
- Qualification: ITI/Diploma Engineering/B.Sc
- Experience: 1–10 years depending on role
- Roles:
- Operate Autoclave/TS, Filling & Capping machines, Vial Washing, and Depyrogenation tunnels.
- Handle Automatic Visual Inspection/X-ray machines (Brevetti, Tofflon, Eisai, Wilco).
- Prepare Knapp/Challenge/Defect Kits.
- Supervisory roles for Filling Line/Aseptic area – Grade B (B.Pharm) including batch manufacturing, autoclave, garment washing, and vial/ampoule washing.
- Preference: Experience in sterile pharmaceutical plants.
Quality Assurance Department
Officer / Sr. Officer / Executive / Sr. Executive
- Qualification: B.Pharm, M.Pharm
- Experience: 2–10 years
- Roles:
- QMS: Handle Quality Management Systems, Change Control, Market Complaints, Risk Assessments, Vendor Qualification, Audits & Compliance, Nitrosamine & elemental impurities assessment.
- Equipment Qualification: Execute equipment, utility, and CSV, conduct media fill studies.
- Validation: Process validation, cleaning validation, APQR.
- IPQA: In-process & finished product sampling, BMR/BPR review, Aseptic Process Simulation, AQL, Artwork review.
- GLP QA: Review QC documents, analytical & microbiology methods validation, instruments handling (UV, HPLC, FTIR, GC, LPC, TOC, Polarimeter, Refractometer), incident management, change control, handling control samples, 21 CFR Part 11 compliance.
- Preference: Candidates with experience in sterile pharma plants.
Eligibility / Qualifications
Relevant Courses: ITI, Diploma in Engineering, B.Sc, B.Pharm, M.Pharm, QA/QC Certification, Sterile Manufacturing Experience.
- Production roles: ITI/Diploma/B.Sc, 1–10 years of experience.
- QA roles: B.Pharm/M.Pharm, 2–10 years of experience.
- Immediate joiners will be preferred.
Location & Salary
- Location: Hotel Deotel, Opp. Hotel Manaal, Mumbai-Agra Highway, Pithampur, Indore.
- Salary: Competitive, commensurate with experience (details shared during interview).
Application Process
Candidates can attend the Walk-In Drive on:
- Date: Saturday, 11th October 2025
- Time: 10:00 AM – 03:00 PM
- Venue: Hotel Deotel, Mumbai – Agra Highway, Opp. Shiv Shakti Petrol Pump, Near Chopati, Opp. Manal Hotel, Pithampur, Indore.
Documents to carry:
- Updated resume
- Passport-size photograph
- Educational certificates
- Last 3 months salary slips, latest appointment letter, and increment letter (if any)
Contact Email: hr.indore@guficbio.com
Act fast to secure your place in this prestigious walk-in recruitment and become a part of Gufic’s high-performance culture, contributing to the future of injectable pharmaceuticals.
FAQs
Q: Who can attend the walk-in drive?
A: Candidates with the relevant qualifications and experience in production or QA/sterile pharma are eligible.
Q: Are fresher candidates considered?
A: Preference will be given to candidates with experience, but freshers with relevant academic projects or internships may be considered for technician roles.
Q: What is the interview process?
A: Walk-in interviews with HR and department heads. Immediate joiners will be preferred.
Q: Is on-the-spot offer possible?
A: Yes, candidates meeting criteria may receive immediate joining offers.
Q: What are the benefits of working at Gufic Biosciences?
A: Competitive salary, professional growth, global exposure, and experience in a world-class sterile pharmaceutical facility.
Apply Now: Prepare your documents and join Gufic Biosciences Walk-In Drive to elevate your pharmaceutical career and contribute to a global leader in injectable medications.
| Category | Details |
|---|---|
| Company | Gufic Biosciences Limited |
| Vacancies | Production Technician/Sr. Technician, Production Officer/Sr. Officer, QA Officer/Sr. Officer/Executive/Sr. Executive |
| Required Education | ITI, Diploma in Engineering, B.Sc, B.Pharm, M.Pharm, QA/QC Certifications, Sterile Manufacturing Experience |
| Experience | Production: 1–10 years, QA: 2–10 years |
To apply for this job email your details to hr.indore@guficbio.com